The procurement of bulk sapphire crystals is the prerequisite for the production of sapphire windows. The dimensions and qualities of the bulk sapphire crystal have to conform to definite criteria according to the requirements of users. Poor qualities of sapphires could lead to severe problems in the sapphire window (e.g. bubble inclusions in the sapphire could cause undesired optical absorption presenting in the window and lattice dislocations might cause depositions during the polishing process of the sapphire window). Therefore sapphire window manufacturers should be careful while fabricating or purchasing raw sapphire materials.
Sapphire is a corundum composed of Aluminum Dioxide (Al2O3) arranged in 3D-regular, periodical, and reduplicated crystalline state of hexagonal or cubic structures. Besides Al2O3, nature sapphire contains substantial amounts of a foreign substance, which is the exact cause of the gorgeous color appearing in sapphire gemstones.
There are 9 kinds of sapphire known so far in total, including gamma sapphire, beta sapphire, alpha sapphire, and eta, zeta, theta, kappa, rho, chi sapphire. The sapphire selected to produce sapphire windows is Alpha Single Crystal Sapphire grown using specific artificial techniques in the labs and factories. These artificial sapphires are also often referred to as “Sapphire Glass” (which could be misleading, because sapphire glass is not a real kind of glass, it exists in crystalline forms rather than amorphous glass forms). Compared with natural sapphire, the grown sapphire or sapphire glass is colorless, and features higher Al2O3 purities, exclusion of water, and more ordered, predictable micro-structures, allowing it to fulfill industrial and optical-grade demands better.
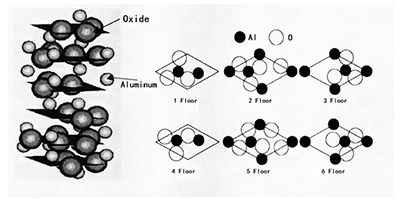
The atomic structure of alpha-sapphire used to produce sapphire windows is shown in the figure below. The left half of the figure shows that alpha-sapphire constitutes hexagonal-closest piled oxide floors, and the 3/2 gaps of the octahedron are filled with Al3+ ions. The right part of the figure illustrates six floors of Al2O3 unit cells arranged in ARAB manners. Each floor contains 3 oxide atoms and 18 oxide atoms in total. Regarding the Al atoms, on the 1 and 4 floors, there are two each, and the remaining 4 floors contain 3 each, so that will be 12 Al atoms in total.